- Home
- Research
- Research Cores and Services
- Pharmacokinetic and Mass Spectrometry Core
- Pre-Clinical Studies
- Academic Departments
- Research
- Academic Departments
- Research in Clinical Pharmacy
- Research in Medicinal Chemistry
- Research in Pharmaceutical Sciences
- Research Cores and Services
- Biointerfaces Institute
- Michigan Drug Discovery
- Michigan Institute for Clinical & Health Research
- Translational Oncology Program
- Faculty Publications
- News
- UM Pharmacy Professor Research Outreach (PRO)
- About the College
Pre-Clinical Studies
We support:
|
|
|
|
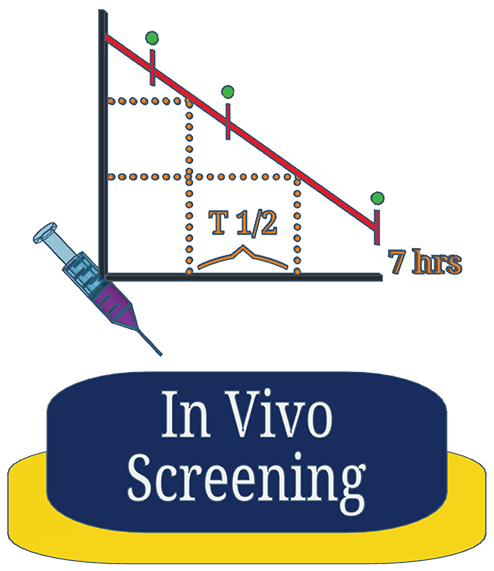
Gain rapid insight into your compounds behavior in vivo when administered by different routes. We use a limited number of animals with selected time-points to provide initial maximum-concentration and half-life estimates.
|
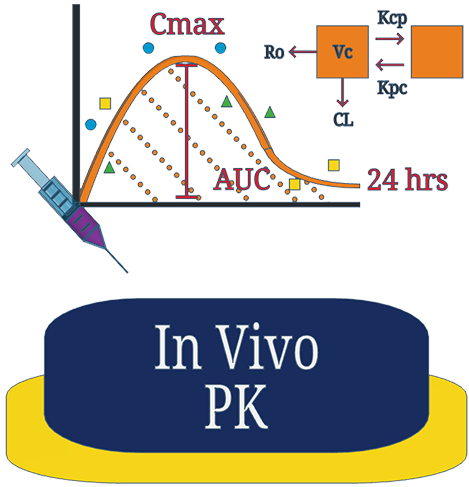
Gain broader insights into your compounds PK profile to support your dose translation in future disease models. We use selected healthy animals at varying time-points to generate a more robust concentration-time profile for clearance and volume of distribution estimates.
|
|
|
|
|
Understanding the oral bioavailability of your compounds can aid in lead selection for further development. We use an intravenous and oral dosing protocol to help define oral bioavailability in rodent models.
|
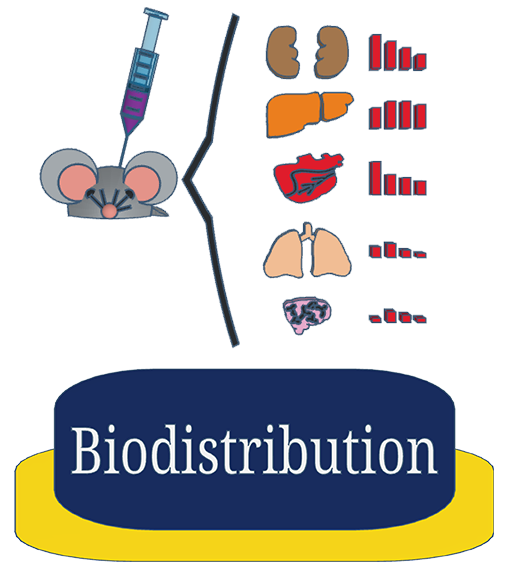
Plasma concentrations may or may not truly reflect target tissue concentrations. We can help you ascertain tissue concentration in a specific tissue of interest and into up to 17 different target organs in rodent models.
|
|
|
|
|
Understanding the acute toxicity of your compound can aid identification of target affected organs and selection of doses for repeated-dose toxicity studies. We use a 5 step single dose escalation protocol to help you identify the maximum tolerated dose in the mouse. |
Understanding the disposition and therapeutic effects of drugs is essential to dose translation. We use a human tumor xenograft model in the immunocompromised mouse to help you understand the pharmacodynamics of your compound. |
Listing Row
- Academic Departments
- Research
- Academic Departments
- Research in Clinical Pharmacy
- Research in Medicinal Chemistry
- Research in Pharmaceutical Sciences
- Research Cores and Services
- Biointerfaces Institute
- Michigan Drug Discovery
- Michigan Institute for Clinical & Health Research
- Translational Oncology Program
- Faculty Publications
- News
- UM Pharmacy Professor Research Outreach (PRO)
- About the College