Research Feature - Timothy Cernak
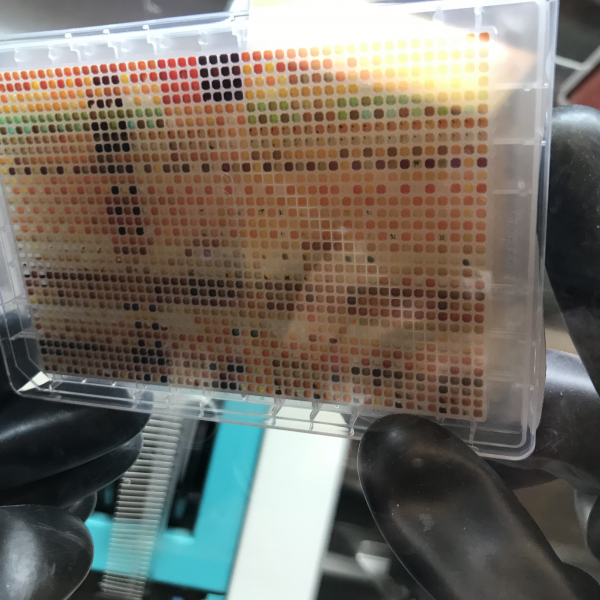
Computers have shrunk in size and grown in power, all due to the magic of miniaturization. You likely have a smartphone in your pocket, which was made possible through miniaturization of transistors. The Cernak Lab in the Department of Medicinal Chemistry is applying the miniaturization concept to chemistry. They are running chemical reactions on a tiny scale, which leads to orders of magnitude more chemical data coming out of every experiment. A series of recent high impact papers in Science and Nature from Timothy Cernak, PhD, Assistant Professor of Medicinal Chemistry, and colleagues at Merck & Co. Inc., pave the way for the application of miniaturized chemistry in drug discovery and beyond.
“The beauty of miniaturization is that it enables parallel processing, so where a typical synthetic chemistry experiment delivers one data point, the miniaturization technique I’ve developed delivers more than one thousand data points. And lots of data is surely needed in the modern era of drug discovery,” explains Prof. Cernak. “What is a medicine? Lipitor, Ibuprofen, Viagra, Gleevec, Sitagliptin….these are all molecules which were synthesized from a near-infinite number of possible molecules which exist, theoretically in what is known as ‘chemical space.’ Searching through chemical space to find that one single molecule, which may be a life-saving drug, is a very difficult task (and cost upwards of a billion dollars).”
By performing chemical synthesis on nanoscale, researchers in the Cernak Lab can tackle problems in chemical synthesis and drug discovery that were previously impossible. “We are able to make molecules that fail when using traditional tactics. Because the miniaturized technology my lab is developing enables access to large swaths of synthesis data, they are able to navigate to needle-in-the-haystack combinations of solvents, oxidants, and transition metal catalysts derived from palladium, nickel, copper or iridium,” notes Prof. Cernak. “Forging a bond in a drug molecule requires just the right recipe of solvents and catalysts to succeed. In the status quo, researchers hunt for successful conditions one reaction at a time, when more than a thousand reactions can be run at once, more successful reaction conditions can be found.”
In one example recently published in Nature, Prof. Cernak and coworkers at Merck & Co., Inc. used the miniaturized approach to navigate to inhibitors of checkpoint kinase 1. “We found several highly potent inhibitors – all with less than 20 nanomolar IC50’s – which could only be found because of our miniaturized approach. The synthesis of these molecules completely failed when a single catalyst condition was used. It was necessary to screen many conditions to find the ones that worked. Think of all of the missed opportunities in drug discovery we are just passing over simply because the designed molecule cannot even be synthesized in the first place,” says Prof. Cernak.
The Cernak Lab is applying the miniaturized approach towards diverse synthetic problems.
“We have built a very modern lab loaded up with robotics and students are blasting through chemical space,” explains Dr. Cernak. “Right now we are actually exploring something highly theoretical – running a thousand reactions in an experiment for several years now has changed my entire perspective of what synthetic chemistry is. We are developing a new field that is as much mathematics as it is chemistry.”
Read more about Prof. Cernak’s research, including recently published papers: "Mapping the dark space of chemical reactions with extended nanomole synthesis" in Science, "MALDI-TOF MS, Pharmaceutical diversification via palladium oxidative addition complexes" also in Science, and "Nanoscale synthesis and affinity ranking" in Nature.



