First Paper Published by the Cernak Lab Featured in Nature

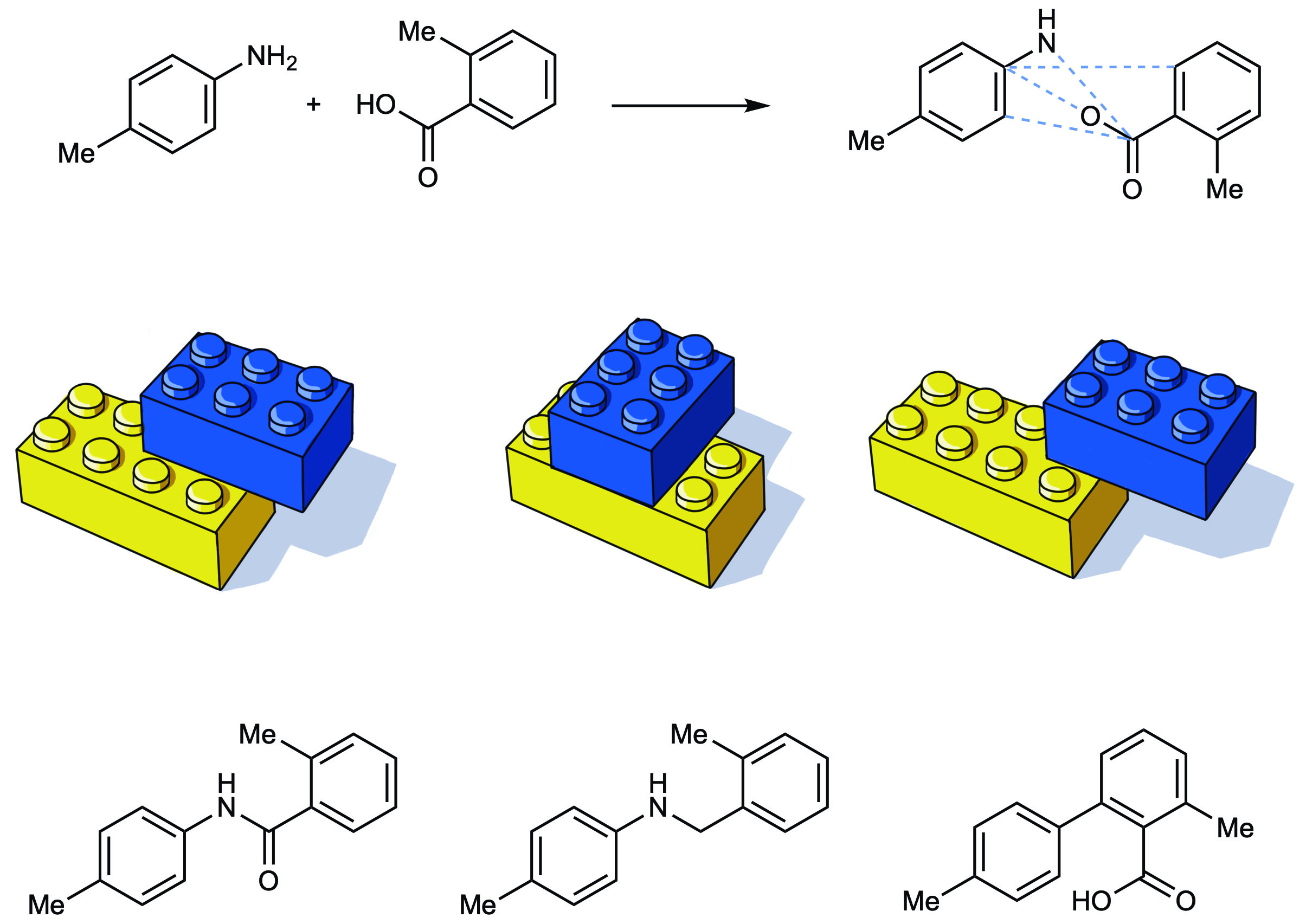
Recently featured in Nature, “A map of the amine-carboxylic acid coupling system” opens the door to a new field of drug discovery. Until now, the coupling of an amine with a carboxylic acid to form an amine bond has been the reaction used in 25% of drug discovery. However, the new paper from the Cernak Lab illustrates that amines and acids can actually couple via hundreds of other transformations, each producing a product with unique properties.
The diversity of properties and molecular shapes achievable with the new method will enable medicinal chemists to fine-tune the properties of drug candidates. In theory, this new layer would allow researchers to choose a desired reaction first and work their way back to the building blocks. Lead author Tim Cernak, Assistant Professor of Medicinal Chemistry, hopes this new dimension will inspire many original chemical bonds that may otherwise have been overlooked. “This research gives us the ability to explore chemical space in an entirely new dimension. By linking chemical reactions to properties, we can design drugs with unique profiles such as increased solubility, metabolic stability, or distribution to specific tissues, like the brain or the heart.”
This is the first paper published by the Cernak Lab, which was established when Dr. Cernak joined the College of Pharmacy’s Department of Medicinal Chemistry in 2018 after spending nearly a decade in big pharma. The lab has been highlighted for their merger of informatics and synthetic chemistry, including the development of robotics for chemistry and chemical algorithms like the one described in the new study. The Cernak Lab aims to build a bridge between data science and chemical synthesis.
Read the full paper HERE.