Pai Lab Research
Alternate body size descriptors for drug dosing. Pharmacokinetic studies often rely on body weight stratification for clinical trials that is based on ideal body weight or body mass index. The scientific basis for this traditional approach has been limited, and more precise descriptors are needed.
A thorough analysis of the literature supported our identification of this rule of thumb known as the ideal body weight equation. This work led to further detailed appraisal of all existing approaches to body size based dosing. Our reliance on Euclidean geometry leads to the mathematical derivation of very similar body surface area equations as a key example. We are working in collaboration with the Morphomic Analysis Group to identify novel body composition metrics that can improve the precision of PK parameter estimates, an approach that we have coined as Pharmacomorphomics.
Crass RL, Ross BE, Derstine BA, Lichty M, Sullivan JA, Su GL, Wang SC, Pai MP. Measurement of skeletal muscle area improves estimation of aminoglycoside clearance across body size. Antimicrobial Agents Chemother. 2018. 62(6). pii: e00441-18. PMID: 29632017
Pai MP, Derstine BA, Lichty M, Ross BE, Sullivan JA, Su GL, Wang SC. Relationships of vancomycin pharmacokinetics to body size and composition using a novel pharmacomorphomic approach based on medical imaging. Antimicrob Agents Chemother. 2017;61(11). pii: e01402-17. PMID: 28807918.
Pai MP, Norenberg JP, Anderson T, Goade DW, Rodvold KA, Telepak RJ, Mercier RC. Influence of morbid obesity on the single dose pharmacokinetics of daptomycin. Antimicrob Agents Chemother. 2007;51(8):2741-2747. PMID: 17548489.
Pai MP and Paloucek FP. The origin of the ‘ideal’ body weight equations. Ann Pharmacother. 2000;34(9):1066-1069. PMID: 10981254.
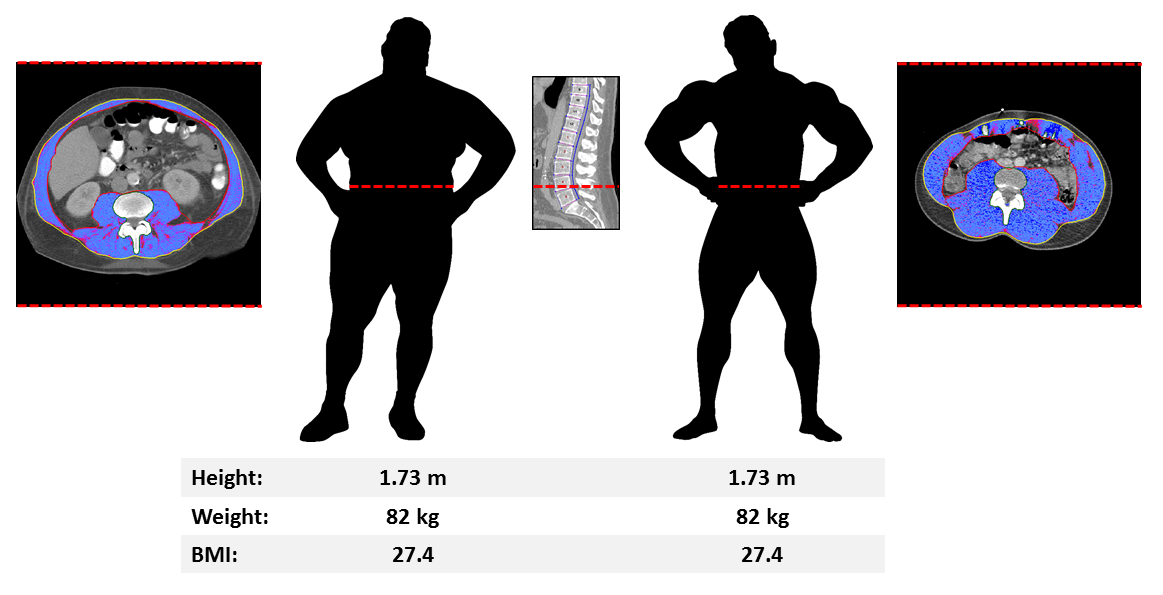
Comparison of two individuals with identical standard body size metrics but strikingly different body composition (skeletal muscle area in blue with red (inner) and yellow (outer) facial boundary at the L4 level) revealed by analytic morphomics.
Antimicrobial pharmacokinetics/pharmacodynamics in obesity. The global body weight distribution has expanded dramatically over the past half-century. The drug development pathway includes clinical trial designs that limit the weight distribution of healthy volunteer participants. This body size restriction leads to the selection of dosage regimens in Phase 3 clinical trials that do not reflect the broader populations. I have served as PI on several Phase 4 clinical trials of antimicrobials in obese healthy volunteers to help validate or question the current labeled dosing regimens.
Hamilton R, Thai XC, Ameri D, Pai MP. Oral bioavailability of linezolid before and after Roux-en-Y gastric bypass surgery: is dose modification necessary in obese subjects? J Antimicrob Chemother. 2013;68(3):666-673. PMID: 23160755.
Pai MP, Lodise TP. Oseltamivir pharmacokinetics in obese adults: dose modification for weight is not necessary. Antimicrob Agents Chemother. 2011;55(12):5640-5645. PMID: 21930887
Pai MP, Norenberg JP, Anderson T, Goade DW, Rodvold KA, Telepak RJ, Mercier RC. Influence of morbid obesity on the single dose pharmacokinetics of daptomycin. Antimicrob Agents Chemother. 2007;51(8):2741-2747. PMID: 17548489.
Design of optimized antimicrobial dosing regimens. Evaluation of the PK/PD profile of antimicrobials in healthy volunteers is a necessary first step to dose definition. However, validation of optimized dosing regimens requires data from the intended target population. My work has focused on evaluation of the antimicrobial PK/PD profile of antimicrobials in acutely ill patients. This work has helped to define novel antimicrobial regimens and adaptive feedback control protocols that are predicted to improve the safety and effectiveness of key antimicrobial agents.
Miglis C, Rhodes NJ, Avedissian S, Kubin CJ, Yin MT, Nelson B, Pai MP, Scheetz MH. Population pharmacokinetics of polymyxin B in acutely ill adult patients. Antimicrob Agents Chemother. 2018;62(3). pii: e01475-17. PMID: 29311071.
Camaione L, Elliot K, Mitchell-Van Steele A, Lomaestro B, Pai MP. Vancomycin dosing in children and young adults: back to the drawing board. Pharmacotherapy. 2013;33(12):1278-1287. PMID: 24019205.
Falcone M, Russo A, Venditti M, Novelli A, Pai MP. Considerations for higher doses of daptomycin in critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57(11):1568-1576. PMID: 24046298.