
Objective: To study the structure, function and biogenesis of intracellular clofazimine crystals and study redox changes upon accumulation of clofazimine in macrophages.
Related Publications:
Macrophages sequester clofazimine in an intracellular liquid crystal-like supramolecular organization. (2012) Baik, J and Rosania GR PlosONE Doi 10.1371/journal.pone.0047494
Multiscale Distribution and Bioaccumulation Analysis of Clofazimine Reveals a Massive Immune System-mediated Xenobiotic Sequestration Response (2012) Baik J, Stringer KA, Mane G, and Rosania GR. Antimicrob. Agents Chemother., doi10.1128/AAC.01731-12
Molecular imaging of intracellular drug-membrane aggregate formation. (2011) Baik J; Rosania GR. Mol PharmaceuticsOct 3;8(5):1742-9.PMCID: PMC3185106
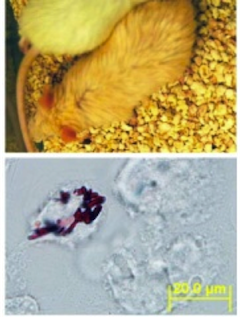
Objective: To study the microdistribution and bioaccumulation of clofazimine in the lungs, in relation to its pro-/anti-inflammatory effects on the airways and alveoli and related changes in lung physiology.
Related Publications:
Multiscale Distribution and Bioaccumulation Analysis of Clofazimine Reveals a Massive Immune System-mediated Xenobiotic Sequestration Response (2012) Baik J, Stringer KA, Mane G, and Rosania GR. Antimicrob. Agents Chemother., doi10.1128/AAC.01731-12
Yu J-Y, Zheng N, Mane G, Min KA, Hinestroza JP, Zhu H, Stringer KA, and Rosania, GR (2012) A cell-based computational modeling approach for developing site-directed molecular probes. PloS Computational Biology. 8(2): e1002378. doi:10.1371/journal.pcbi.1002378. PMC number in progress.
Madathilparambil VS. Wagner MC, Rosania GR, Stringer KA, Min KA, Risler L, Shen DD, Georges GE, Reddy AT, Parkkinen J, and Reddy RC (2012) Pulmonary Administration of Water-soluble Curcumin Complex Reduces ALI Severity.American Journal of Respiratory Cell and Molecular Biology. doi:10.1165/rcmb.2011-0175OC PMC number in progress.


