LABORATORY
Hertz Laboratory

Daniel Hertz, PharmD, PhD
Daniel L Hertz received a PharmD from the Ernest Mario School of Pharmacy at Rutgers University in 2008 and a PhD in Pharmaceutical Sciences from the UNC Eshelman School of Pharmacy in 2013. He has always had a passion for cancer research. During his PhD, Dr. Hertz worked with Dr. Howard McLeod, who introduced him to the field of cancer pharmacogenetics. Dr. Hertz’s dissertation research focused on discovery and validation of pharmacogenetic predictors of taxane-induced neuropathy. After receiving his PhD, Dr. Hertz joined the Department of Clinical Pharmacy at the University of Michigan College of Pharmacy where he is currently an Associate Professor.
Dr. Hertz’s current research is interested in developing tools for individualizing treatment in patients with cancer and translating them into clinical practice. His NCI R37 uses data and biospecimens collected on the prospective SWOG S1714 clinical trial to discover biomarkers (e.g., kinetics, genetics, metabolomics, nutrients) of taxane-induced peripheral neuropathy that can be used to identify patients at higher risk of neuropathy, in whom personalized treatment approaches or experimental preventive strategies can be investigated. He is also an advocate and researcher in the area of DPYD testing to prevent severe fluoropyrimidine toxicity. He is a Medical Advisor to the Advocates for Universal DPD/DPYD Testing and has published several manuscripts describing the clinical utility of pre-treatment DPYD testing.
Accepting PhD Students.
Lab and Research Overview
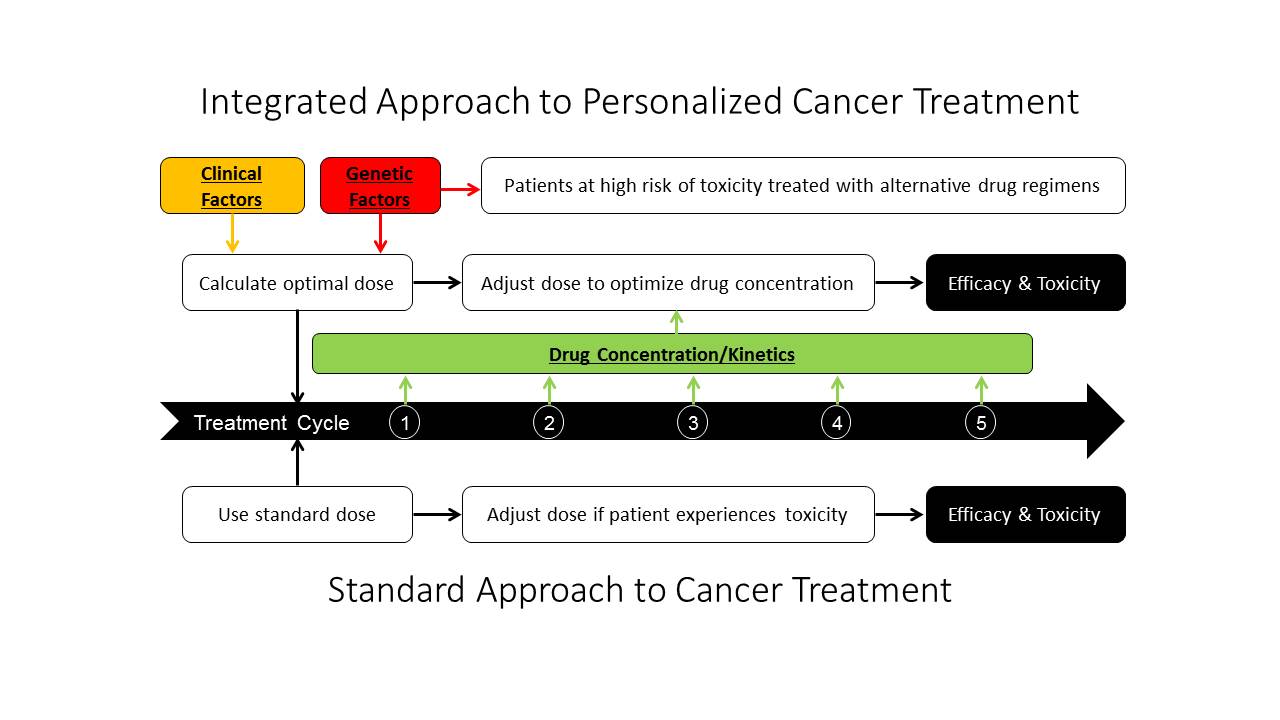
The objective of the Hertz lab is to develop personalized cancer treatment approaches and translate them into clinical practice to optimize therapeutic outcomes. Our research spans the translational spectrum from discovery through implementation. We identify clinical, kinetic, genetic, and physioglocial predictors of cancer treatment efficacy and/or toxicity in retrospective correlative analyses. These discoveries are integrated into novel tools that inform treatment decisions for the individual patient. These tools can then be tested in prospective clinical trials to demonstrate that personalized cancer treatment enhances efficacy and/or prevents unnecessary toxicity.
My lab is accepting PhD students.
Lab Members
Javier Granados II
Teaching & Resources